Which Neutral Atom Has the Following Electron Configuration: 1s22s22p63s23p64s2
Figure 161 shows that the first electron to be removed has the lowest ionisation energy and is therefore the easiest to remove. As each successive electron is removed these ionisation energies can be measured.
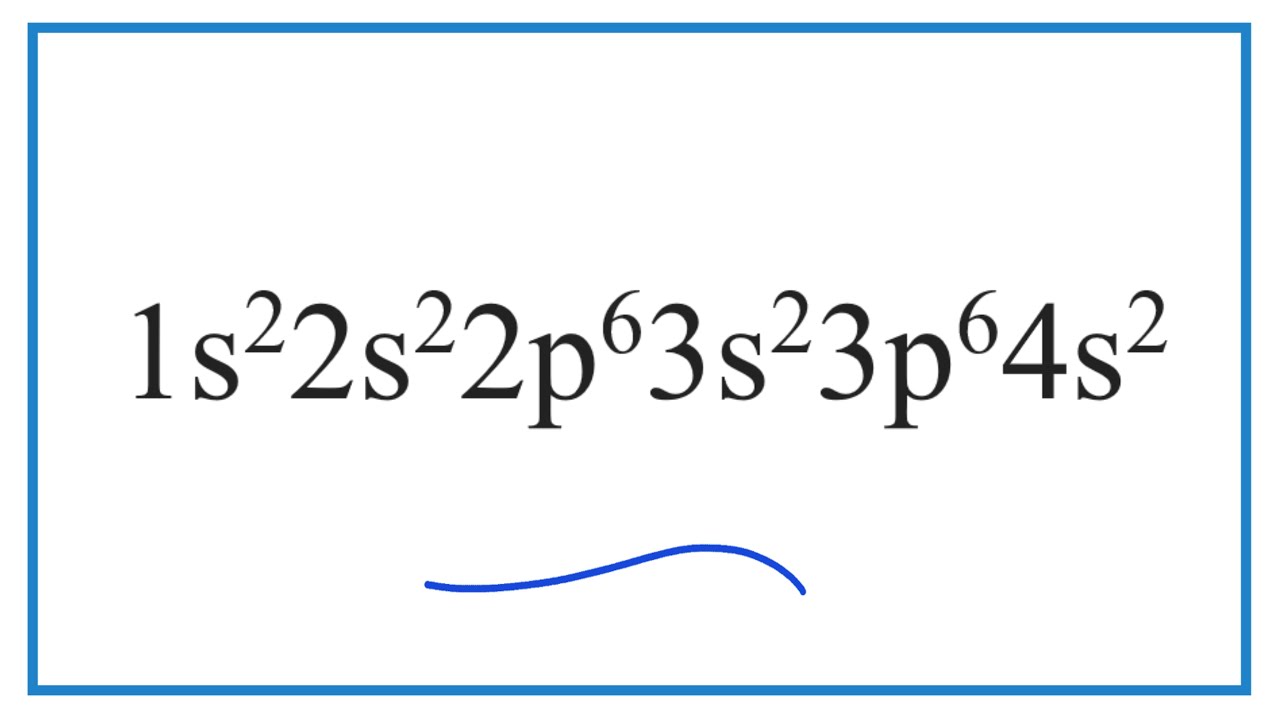
Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s2 Youtube
Calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 1.

. For example a sodium atom contains 11 electrons. One would predict that the element potassium has the following property. The ionisation energy is the energy needed to remove an electron from an atom.
More active than lithium. Element Electron Configuration of Atom Predicted Ion Charge beryllium 1s2 2s2 2 oxygen 1s2 2s2 2p4 3s2.
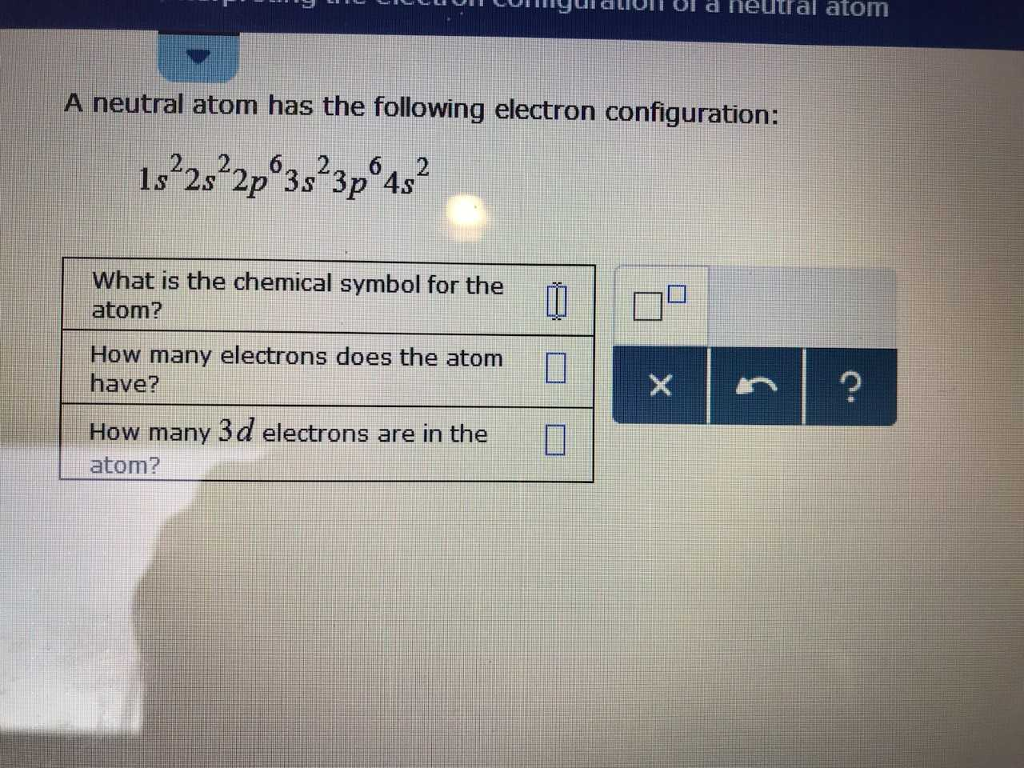
Solved Nguiation Of D Neutral Atom A Neutral Atom Has The Chegg Com

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s1 Youtube

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 Youtube
No comments for "Which Neutral Atom Has the Following Electron Configuration: 1s22s22p63s23p64s2"
Post a Comment